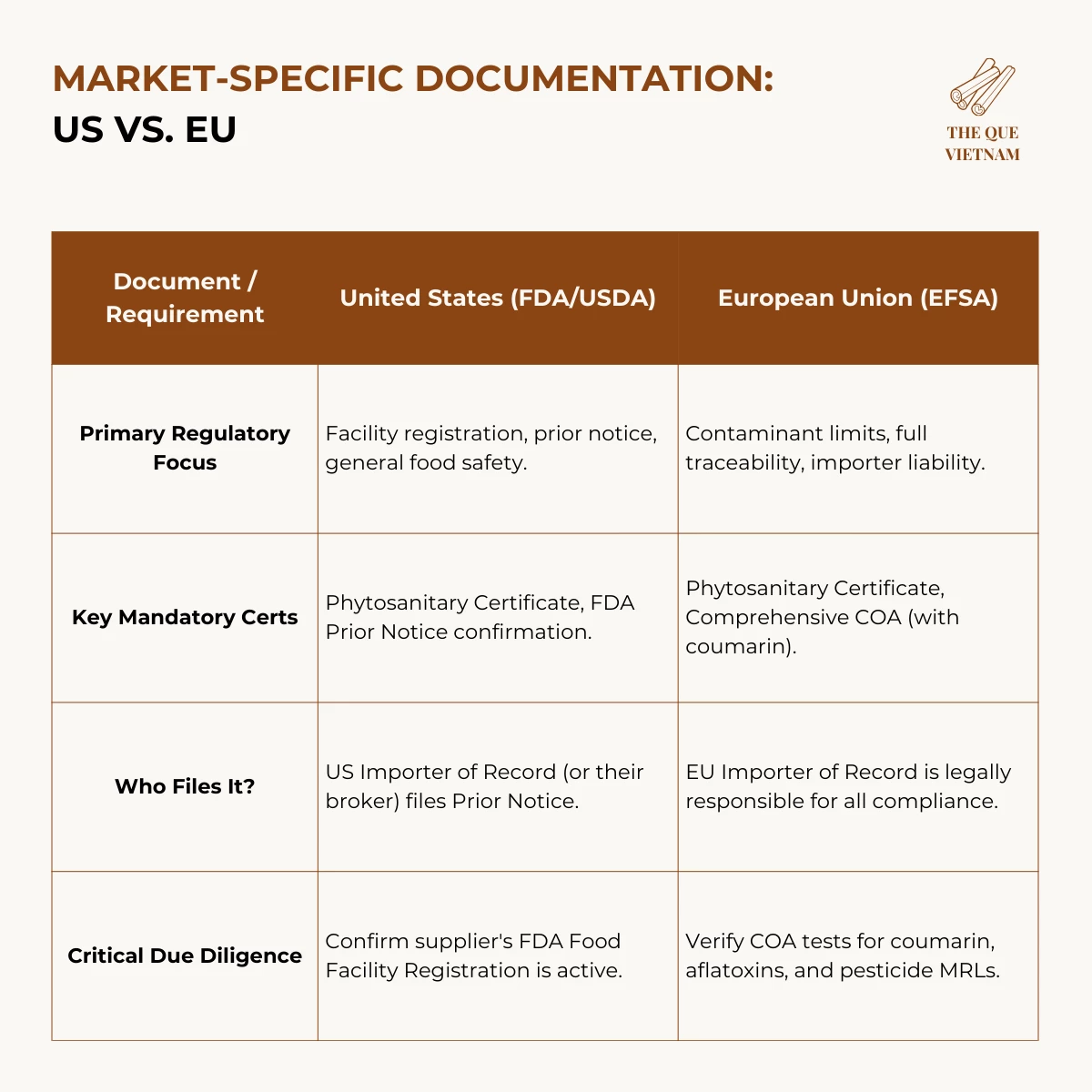
To export cinnamon to the US and EU, importers must ensure FDA facility registration (US), Prior Notice submission, phytosanitary certification, and batch-specific Certificates of Analysis covering moisture, coumarin, aflatoxins, and pesticide residues. EU imports additionally require strict compliance with coumarin limits and full traceability under EC 178/2002.
The Complete Guide to US & EU Cinnamon Export Requirements for Importers
For importers, sourcing premium Vietnamese cinnamon involves more than quality and price—it demands navigating complex food safety regulations to prevent costly delays, rejections, or legal issues. Compliance isn’t bureaucratic; it’s a core component of supply chain reliability.
This guide details the specific export requirements for cassia cinnamon entering the United States and European Union, providing importers with the checklist needed to verify suppliers and ensure seamless market entry.
1. Why Compliance is a Critical Sourcing Filter
Cinnamon is regulated as a food product and plant commodity. Non-compliance doesn’t just cause delays—it can result in port detention, destruction of goods, financial loss, and even blacklisting of suppliers. For buyers, partnering with an export-ready supplier is the most effective risk mitigation strategy, transforming compliance from a hurdle into a competitive advantage.
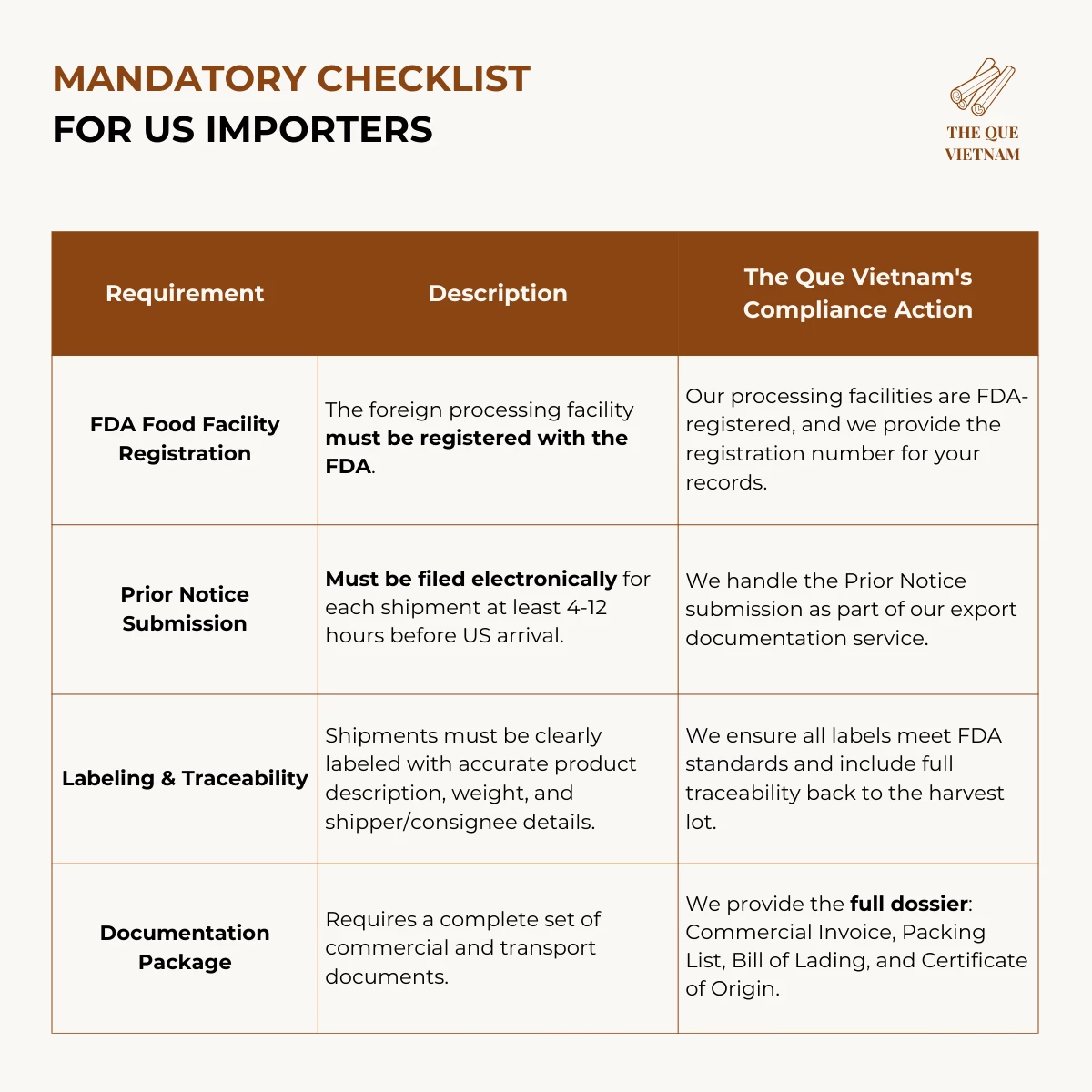
2. US cinnamon export requirements: FDA & Customs Requirements
Importing cinnamon into the US requires adherence to regulations enforced by the Food and Drug Administration (FDA) and US Customs and Border Protection (CBP).
For US imports, compliance centers on FDA registration, shipment notification, and accurate labeling — not product pre-approval.
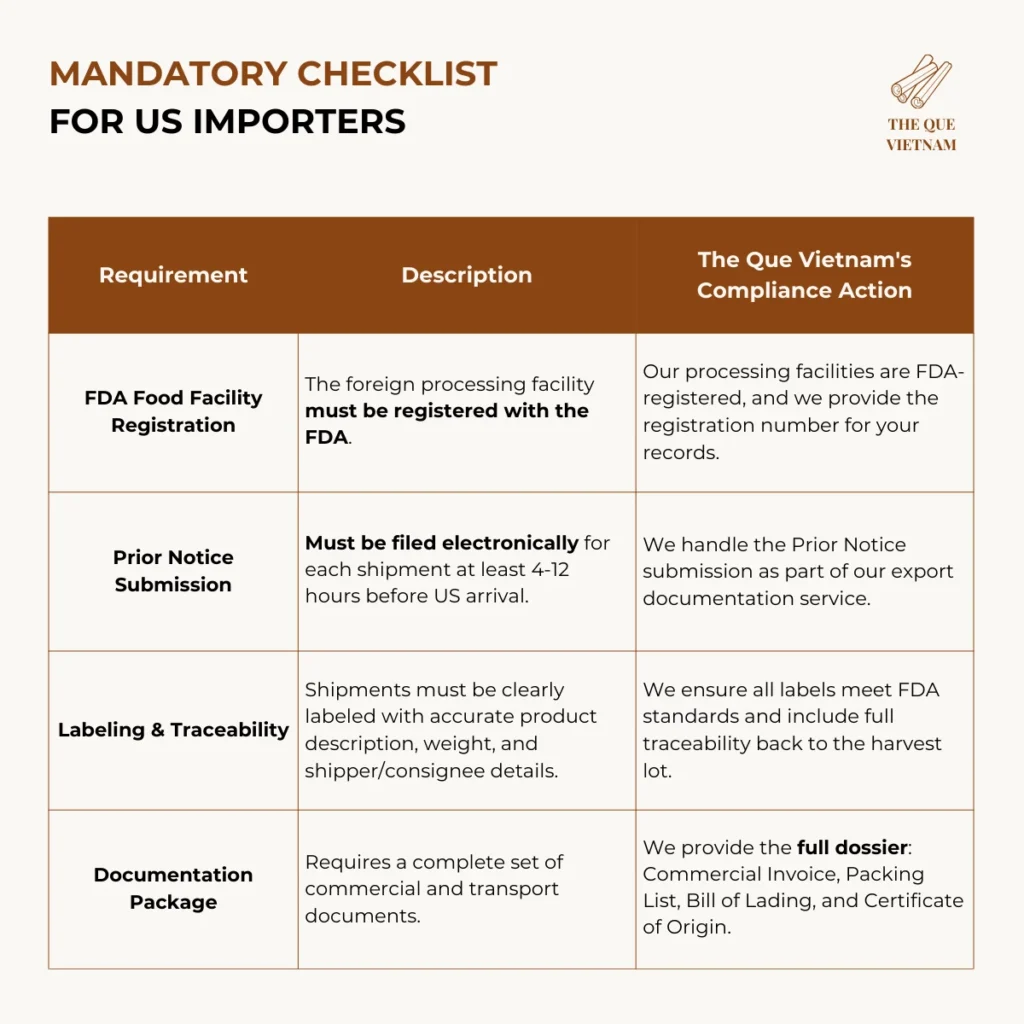
Key US Focus: The FDA prioritizes preventative controls for food safety. While no specific approval is needed for cinnamon itself, the agency can detain shipments if they appear adulterated, misbranded, or from an unregistered facility.
3. EU cinnamon import regulations: Strict Contaminant Limits & Traceability
EU food safety regulations (under EC 178/2002) are among the world’s strictest, emphasizing prevention, traceability, and importer liability.
For the EU, chemical compliance (coumarin, aflatoxins) and traceability are the primary causes of border rejections.
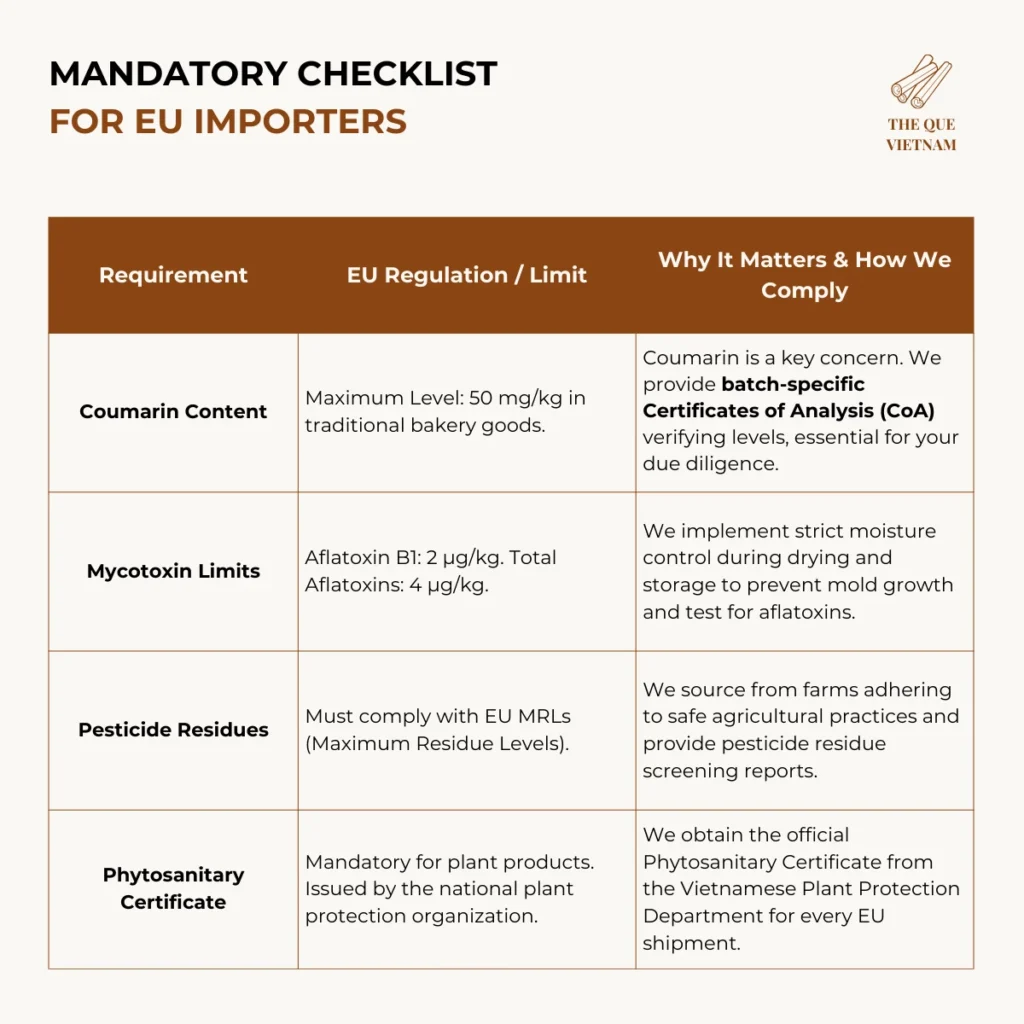
The “Importer of Record” Liability: Under EU law, you, the importer, bear primary responsibility for ensuring the product complies with all regulations. Your strongest safeguard is working with a supplier who provides the verifiable documentation listed above.
Internal Link: For details on coumarin limits and the quality parameters behind these tests, see our guide on Saigon Cinnamon Specifications Explained.
4. Common Compliance Pitfalls & How to Avoid Them
Most shipment delays stem from preventable documentation and specification errors.
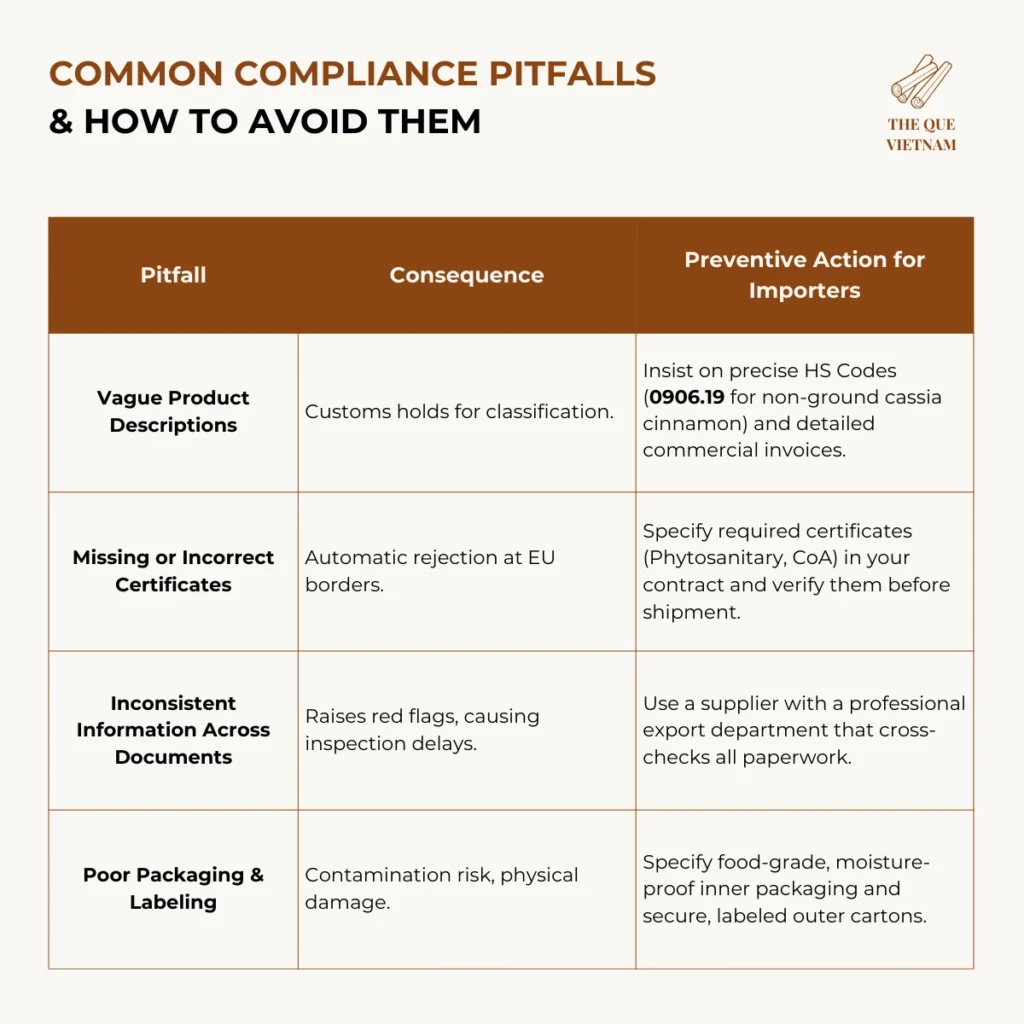
5. Your Supplier Verification Checklist
Before finalizing a contract, use this list to vet your exporter:
-
Can they provide a valid FDA Facility Registration number? (For US)
-
Do they offer batch-specific CoAs for coumarin, aflatoxins, and pesticide residues? (For EU)
-
Who handles the Phytosanitary Certificate and Prior Notice submission?
-
Can you see a sample of their export documentation pack?
-
Do their processing protocols align with international food safety standards (HACCP principles)?
Partner with an Exporter Who Manages Compliance for You
Navigating US FDA and EU food safety law is complex. Your cinnamon should meet the highest standards of quality and paperwork.
At The Que Vietnam, we provide a seamless, compliant export service. You focus on your business; we ensure your cinnamon arrives without regulatory incident.
📥 Request Your Compliance Documentation Pack
Contact The Que Vietnam to:
-
Receive samples of our FDA Registration, Phytosanitary Certificate, and a typical CoA.
-
Get a consultation on your target market’s specific requirements.
-
Obtain a compliant quotation for your next shipment.
Follow The Que Vietnam on Linkedin & Facebook for most updated news!
FAQs: Answering Key Importer Questions
A: The Phytosanitary Certificate is the mandatory entry ticket. However, the Certificate of Analysis for coumarin is equally critical for compliance upon arrival. Never accept a shipment without both.
A: No. Compliance is the result of deliberate processes, registration, and testing. It is your responsibility to verify your supplier’s compliance, not assume it.
A: Testing (for coumarin, aflatoxins) and certification add to the product cost, but they are non-negotiable for market access. Consider them essential insurance against far greater losses from a rejected shipment.
A: We build it into our process. From FDA registration and HACCP-based processing to partnering with accredited labs for testing and managing all export documentation in-house, we ensure your shipment is prepared correctly from the start.