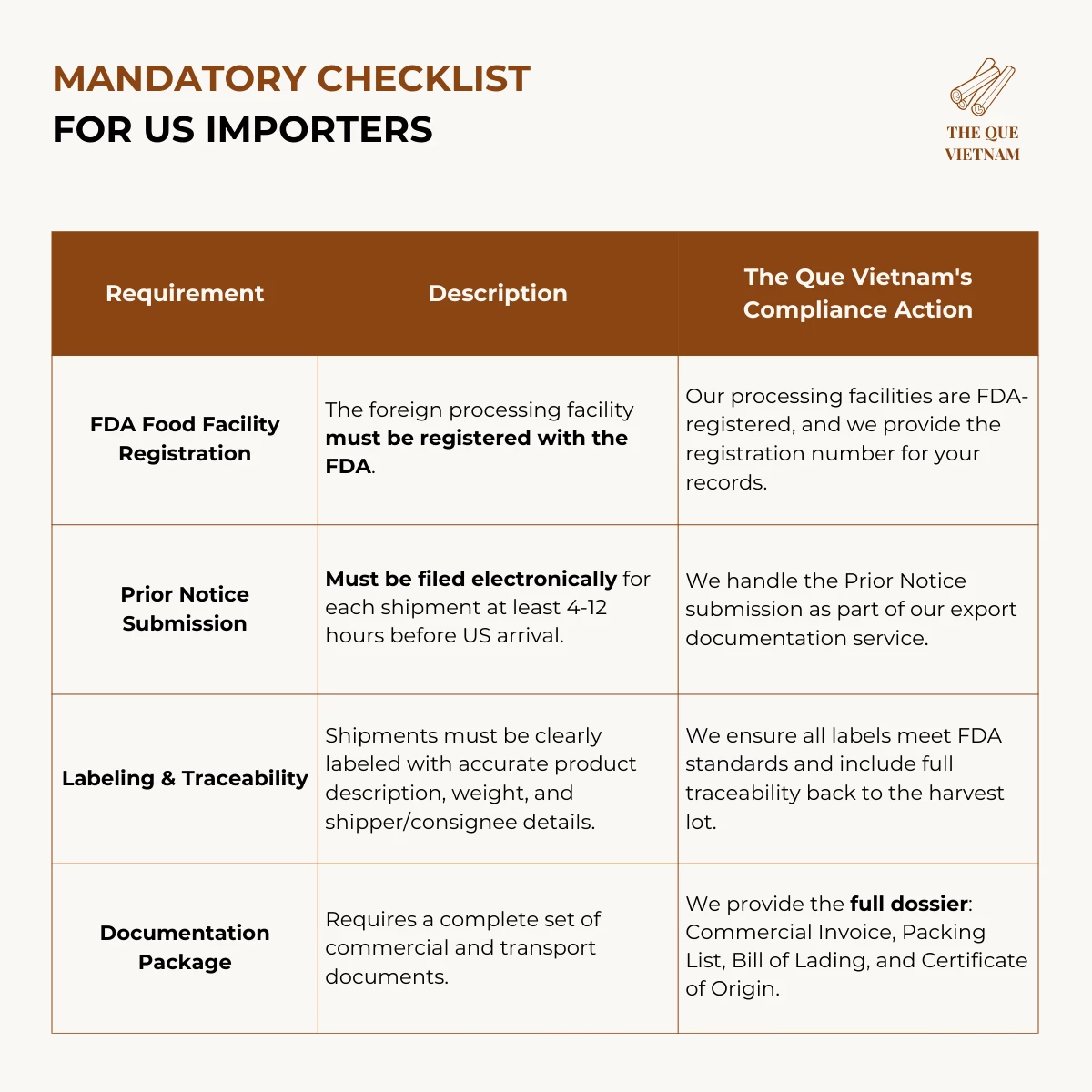
To import cinnamon into the US and EU, buyers must obtain a Certificate of Origin, Phytosanitary Certificate, and batch-specific Certificate of Analysis (COA). US imports also require FDA facility registration and Prior Notice, while EU imports require coumarin, aflatoxin, and pesticide residue testing for compliance.
Cinnamon Import Documents Demystified: A Checklist for US & EU Importers
For professional importers, export documentation is your shipment’s passport—and your primary risk control tool. Incorrect or missing paperwork is the #1 cause of costly customs holds, port delays, and rejected shipments.
This guide details every essential document required to import Vietnamese cassia cinnamon into the United States and European Union, explaining what to look for, why it matters, and how to spot red flags before your container sails.
1. The High Stakes of Export Documentation
Cinnamon shipments are scrutinized as both a food product and an agricultural commodity. Documentation isn’t administrative; it’s legal proof of safety, origin, and compliance. Incomplete paperwork can trigger:
-
Customs holds (demurrage fees: $100-$300/day)
-
Physical inspection and sampling (delays: 7-14+ days)
-
Complete rejection and forced re-export
Working with a supplier who treats documentation as a core competency is your first line of defense.
Internal Link: For the regulatory context behind these documents, see our comprehensive guide on Cinnamon Export Requirements for US & EU Importers.
2. The Indispensable Core: Three Non-Negotiable Certificates
Every compliant shipment is built on these three foundational documents.
A. Certificate of Origin (CO)
-
Purpose: Legally certifies the product’s country of manufacture (Vietnam). Determines applicable import duties under trade agreements.
-
What to Verify:
-
Issued by a recognized chamber of commerce in Vietnam.
-
Exporter/Consignee details match your commercial invoice exactly.
-
Clearly states “Country of Origin: Vietnam.”
-
-
Red Flag: A generic, non-chamber-issued document may be rejected by customs.
B. Phytosanitary Certificate
-
Purpose: Official government guarantee that the shipment has been inspected and is free from regulated pests and soil. Mandatory for plant products entering the US and EU.
-
What to Verify:
-
Issued by the Vietnam Plant Protection Department (or equivalent NPPO).
-
Includes the correct botanical name (Cinnamomum cassia or Cinnamomum loureiroi).
-
The “Place of Origin” field lists specific growing regions (e.g., Yen Bai Province).
-
-
Red Flag: A certificate issued long before the shipment date may raise questions about its validity for that specific batch.
C. Certificate of Analysis (COA) – Your Quality Proof
The COA is your technical assurance. A professional COA from an accredited lab should report:
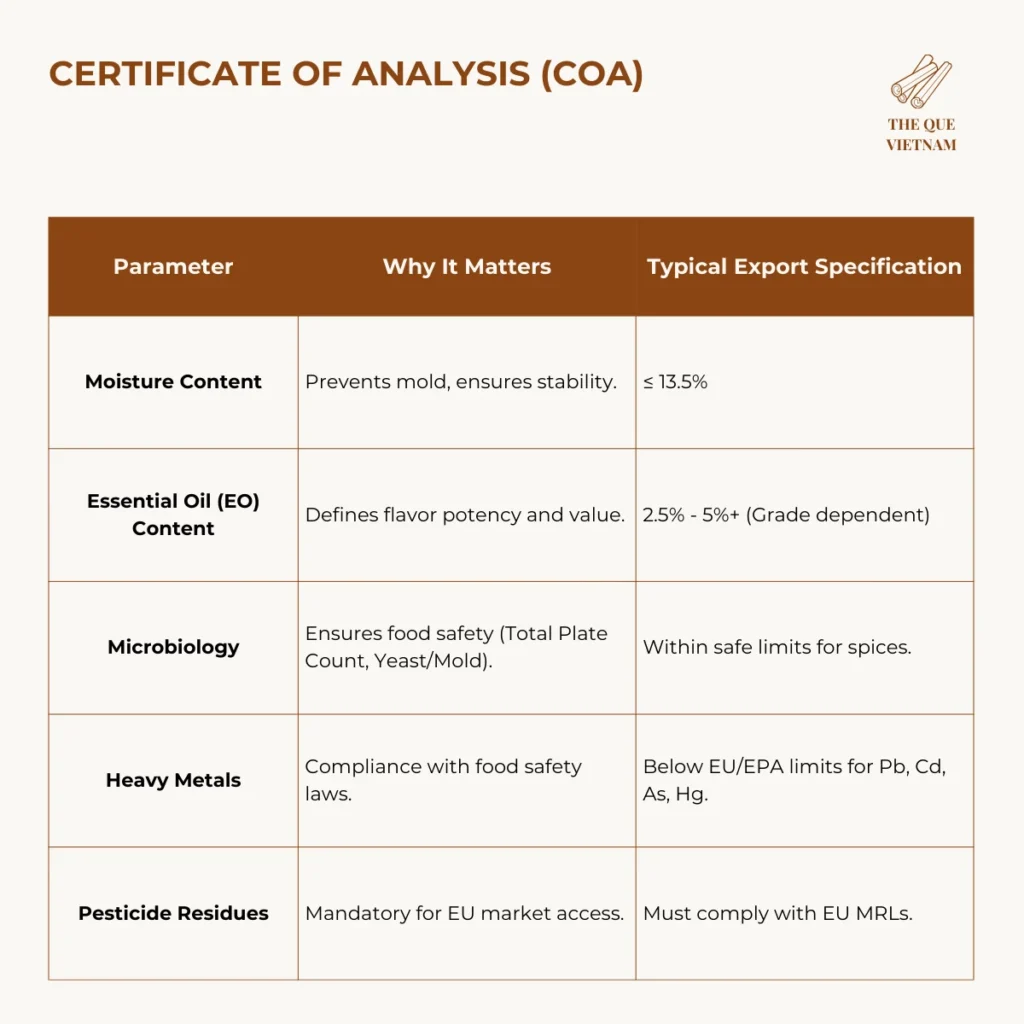
Crucial for EU Buyers: The COA must include coumarin levels to prove compliance with the 50 mg/kg limit in traditional foods.
For EU-bound shipments, laboratories should be ISO/IEC 17025 accredited (e.g., SGS, Eurofins, Intertek) to ensure results are accepted by border authorities.
These three documents are mandatory for customs clearance in both the US and EU.
3. The Complete Export Dossier: Supporting Documents
A professional shipment includes these additional documents for a seamless process:
-
Commercial Invoice: The financial cornerstone. Must match the Packing List and Bill of Lading in weight, value, and description.
-
Packing List: Detailed breakdown of each package (weight, dimensions). Critical for customs and logistics.
-
Bill of Lading (B/L): The title of goods and contract with the shipping line. Verify “Shipper” and “Consignee” details meticulously.
-
Fumigation Certificate: Often required if wooden pallets or crates are used, certifying they are pest-free.
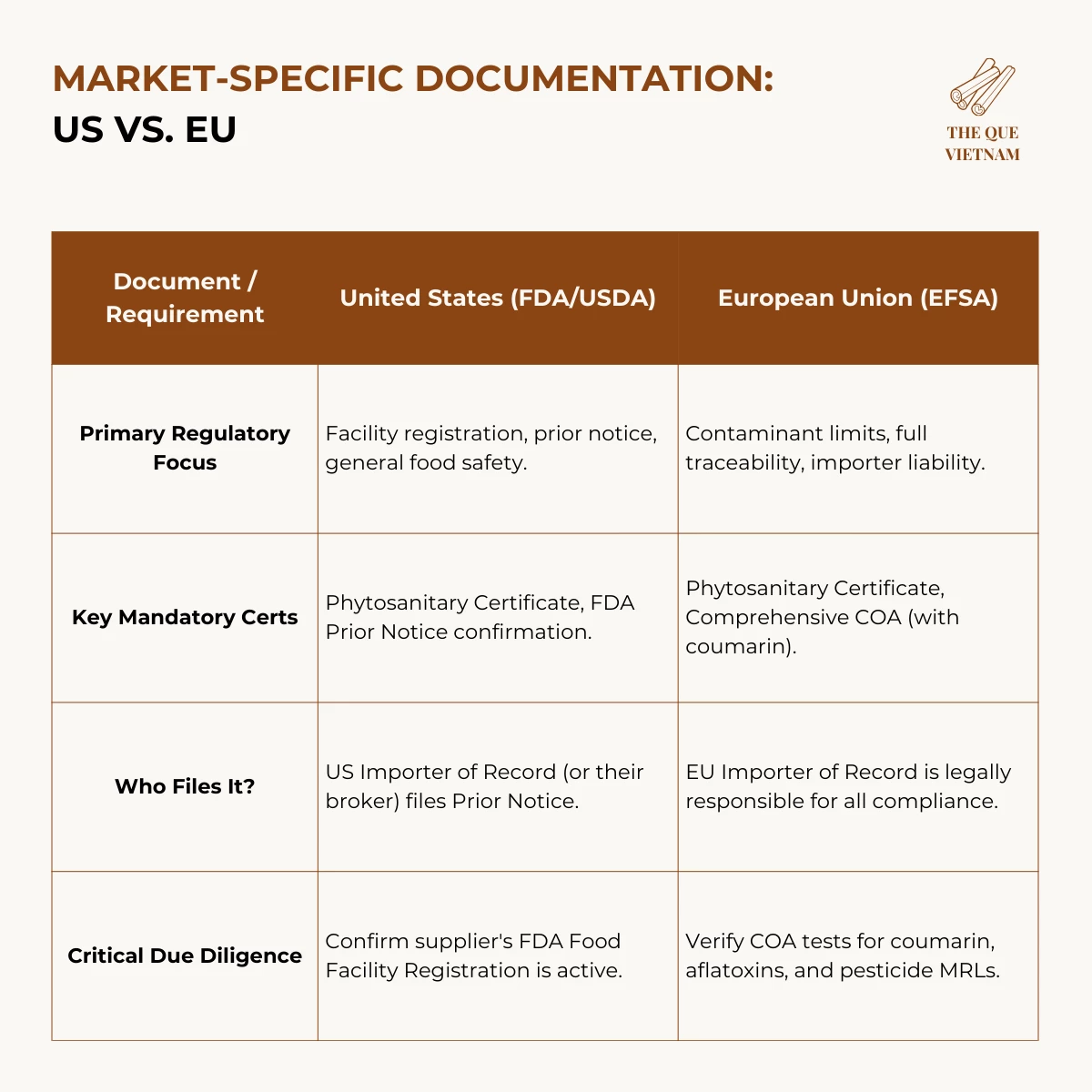
4. Market-Specific Documentation: US vs. EU
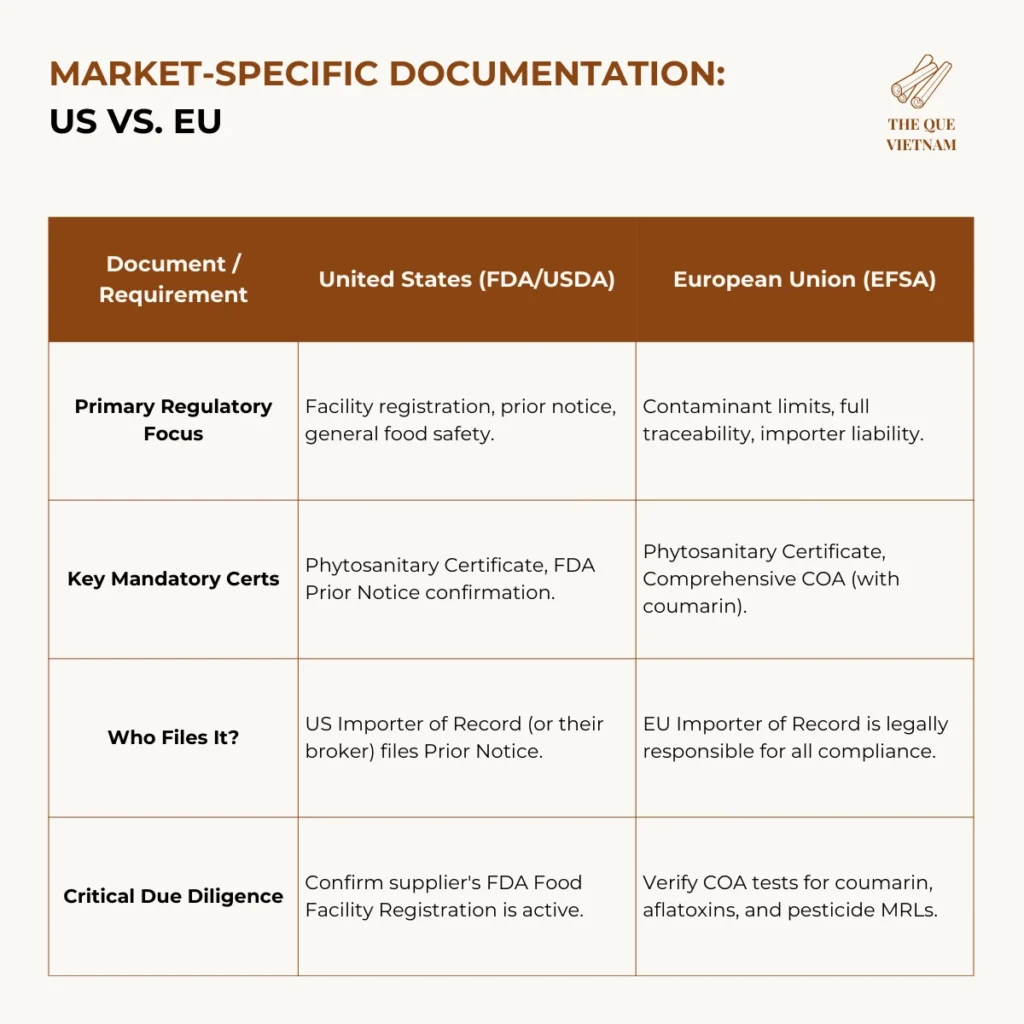
US compliance focuses on facility registration and shipment notification, while the EU emphasizes chemical safety and importer liability.
5. Your Pre-Shipment Document Audit Checklist
Protect yourself by reviewing samples before production. Email your supplier this list:
-
Certificate of Origin (CO) Sample: Is it from a Vietnamese Chamber of Commerce?
-
Draft COA Template: Does it test for ALL required parameters (especially coumarin for EU)?
-
Phytosanitary Certificate Sample: Is it from the official Plant Protection Department?
-
Commercial Invoice & Packing List Drafts: Do weights, descriptions, and HS Codes (0906.19 for cassia cinnamon) match perfectly?
-
FDA Registration Number (for US): Can they provide it?
The Golden Rule: Consistency is key. The product description (e.g., “Saigon Cinnamon Quills, Grade A”) and weights must be identical across ALL documents.
Partner with a Supplier for Whom Documentation is a Priority
Your cinnamon should be as impeccable on paper as it is in the bag. Accurate documentation is the hallmark of a serious, professional exporter.
At The Que Vietnam, we don’t just send documents—we build compliant, traceable export dossiers that give you and customs authorities complete confidence.
📄 Request a Sample Export Document Pack
Contact The Que Vietnam to:
-
Receive authentic samples of our Certificate of Origin, Phytosanitary Certificate, and a detailed COA.
-
Get a documentation consultation tailored to your first port of entry.
-
Secure a quotation from an exporter that prioritizes compliant, hassle-free logistics.
Follow The Que Vietnam on Linkedin & Facebook for most updated news!
FAQ: Solving Common Importer Document Dilemmas
A: The exporter (supplier) is responsible for providing the Certificate of Origin, Phytosanitary Certificate, and COA. The importer (you) or your customs broker is responsible for submitting Prior Notice (US) and ensuring the full dossier meets your country’s rules. A professional exporter will guide you.
A: No. This is a major red flag. A legitimate COA must be batch-specific, linked to the exact lot you are purchasing, with a unique sample ID and testing date. Reject generic COAs.
A: Mismatched information. For example, the weight on the Bill of Lading is 20,100 kg, but the commercial invoice says 20,000 kg. Customs automation flags these discrepancies instantly, causing manual reviews and delays.
A: We have a dedicated export documentation team. We provide a complete, pre-shipment document pack for your review and assign a unique batch number to your order that links your product, its lab tests (COA), and all export certificates, guaranteeing full traceability and consistency.